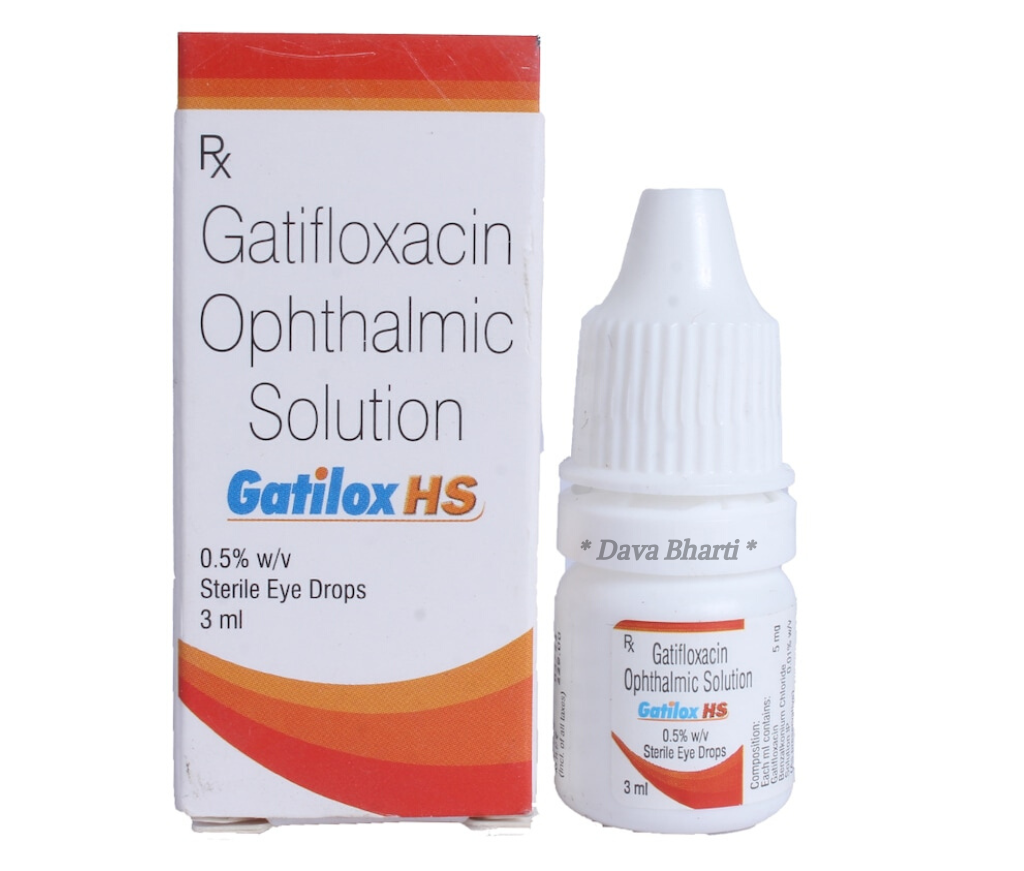
Gatifax -HS Eye Drop
![]() Item requires a valid prescription
Item requires a valid prescription
Manufactured By : Optho Remedies Pvt Ltd
Composition : Gatifloxacin Topical (0.5% w/v)
Rs 92.14
MRP Rs 115.17
(20% OFF)
Includes all taxes
Package SIZE
( 5 ml in 1 packet )
100% Authentic
Products
Free
Shipping*
Product
Returnable
- Uses / Indications: Gatifax -HS Eye Drop is used in the treatment of bacterial infections.
Interactions / Warnings: No interaction found
- Pregnancy interaction:
- Gatifax -HS Eye Drop may be unsafe to use during pregnancy.Animal studies have shown adverse effects on the foetus, however, there are limited human studies. The benefits from use in pregnant women may be acceptable despite the risk. Please consult your doctor.
- Expert advice:
- Your doctor has prescribed Gatifax to cure your infection and improve symptoms. Do not skip any doses and finish the full course of treatment even if you feel better.
- How to use:
- This medicine is for external use only.Take it in the dose and duration as advised by your doctor. Check the label for directions before use. Hold the dropper close to the eye/ear without touching it. Gently squeeze the dropper and place the medicine inside the lower eyelid or ear. Wipe off extra liquid.
- How it works:
- Gatifax -HS Eye Drop is an antibiotic. It kills bacteria by inhibiting the DNA replication.
- Faq for medicine:
- Does gatifloxacin topical contain sulfa? : Gatifloxacin topical does not contain sulfa. It is a fluoroquinolone antibiotic|Is gatifloxacin topical the same as Zymaxid? : Zymaxid is the brand name for gatifloxacin eye solution|Is gatifloxacin topical the same as ofloxacin? : Both gatifloxacin and ofloxacin are fluoroquinolone antibiotics and have the same mechanism of killing bacteria. Ofloxacin topical is used to treat eye and ear infections, whereas gatifloxacin is used in the form of eye/ear drops for the treatment of eye and ear infections only. They are also different with respect to the side-effects they cause|Is gatifloxacin topical banned in India? : Gatifloxacin is banned in India for oral/injectable use in humans, however its use as eye/ear drops is not banned.